 The sterilization room is an essential component of the endoscopy suite. Most often, endoscopy material is sterilized right next to the operating room in sub-optimal conditions. We have found it best to set apart a room specifically designed for this purpose, in order to sterilize and store all of our equipment. Besides the risk of infection, there is always a chance that the equipment will deteriorate prematurely if not properly sterilized and stored. All modern endoscopic materials, rigid or flexible, and their attendant accessories must be fully immersible. Current guidelines, inspired by problems encountered in the past with HIV, require immersion of the endoscopes and accessories for 20 minutes after each procedure. The equipment must then be rinsed with sterile water, dried, and stored.
The sterilization room is an essential component of the endoscopy suite. Most often, endoscopy material is sterilized right next to the operating room in sub-optimal conditions. We have found it best to set apart a room specifically designed for this purpose, in order to sterilize and store all of our equipment. Besides the risk of infection, there is always a chance that the equipment will deteriorate prematurely if not properly sterilized and stored. All modern endoscopic materials, rigid or flexible, and their attendant accessories must be fully immersible. Current guidelines, inspired by problems encountered in the past with HIV, require immersion of the endoscopes and accessories for 20 minutes after each procedure. The equipment must then be rinsed with sterile water, dried, and stored.
Disposable equipment
 This equipment is ideal but not always available.
This equipment is ideal but not always available.
The forceps, suction catheters, and stents for example are never recycled.
Autoclavable materials
 Most of the rigid bronchoscopes currently available in the market and their accessories can be autoclaved. This process, however, is time-consuming.
Most of the rigid bronchoscopes currently available in the market and their accessories can be autoclaved. This process, however, is time-consuming.
Some of the equipment, in particular the delicate telescopes must be kept separate and stored in special cases designed to protect them.
Non-autoclavable materials
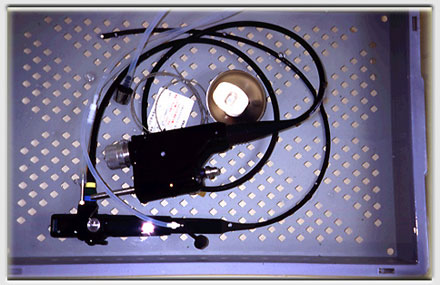 At our endoscopy center, we have developed a standardized sterilization process for the non-autoclavable materials.
At our endoscopy center, we have developed a standardized sterilization process for the non-autoclavable materials.
The endoscopes never leave a special basket.
These baskets are immersed successively in a cleaning tray, followed by a sterilization tray, a rinsing tray with sterile water, and then dried.
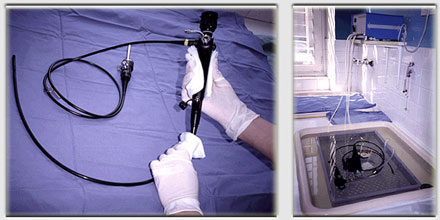 Cleaning is performed by hand, followed by immersion in the tray containing paracetic acid, where mechanical circulation of the disinfectant allows for decontamination of the operating channels of the endoscopes.
Cleaning is performed by hand, followed by immersion in the tray containing paracetic acid, where mechanical circulation of the disinfectant allows for decontamination of the operating channels of the endoscopes.
 When the sterilization process is completed, the endoscopes are kept in their basket, placed in sterile bags, and stored in a cabinet.
When the sterilization process is completed, the endoscopes are kept in their basket, placed in sterile bags, and stored in a cabinet.
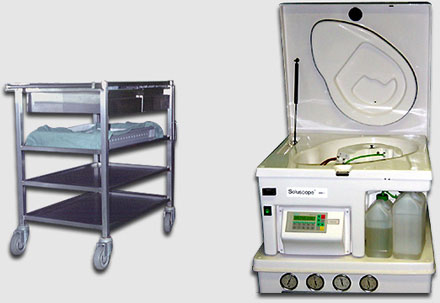 Specially designed carts allow for safe transport of up to three sterilization baskets with their corresponding bronchoscopes to the endoscopy suite.
Specially designed carts allow for safe transport of up to three sterilization baskets with their corresponding bronchoscopes to the endoscopy suite.
The use of a washer simplifies the process, but does not eliminate the all-important first step.
Tracing
 Adequate tracing is essential to optimal quality control. It mandates that everything that goes on in the endoscopy suite must be described in detail and carefully documented in case problems arise.
Adequate tracing is essential to optimal quality control. It mandates that everything that goes on in the endoscopy suite must be described in detail and carefully documented in case problems arise.
Adequate tracing protects the physician and the patient, and documents adherence to established protocols and procedures.
In an endoscopy suite, tracing includes patient monitoring, equipment sterilization and maintenance procedures, physical plant maintenance, and quality control of all interventions taking place in the center.
 Tracing all aspects of patient care
Tracing all aspects of patient care
 Concerning the patient, careful documentation of all of the following elements is essential prior to any procedure ; name and last name, date of birth, date of hospitalization, admitting service, medical record number, referring physician, informed consent signed by the patient, as well as documentation of Creutzfeld-Jacob disease risk-assessment signed by the referring physician.
Concerning the patient, careful documentation of all of the following elements is essential prior to any procedure ; name and last name, date of birth, date of hospitalization, admitting service, medical record number, referring physician, informed consent signed by the patient, as well as documentation of Creutzfeld-Jacob disease risk-assessment signed by the referring physician.

- Protocol (Traceability concerning patients)
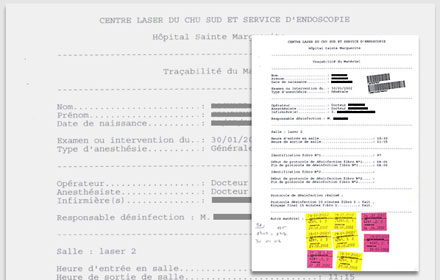 Concerning the procedure itself, careful instructions are provided which have medical-legal value, and are placed in the patient´s records. A copy is always kept and stored in the endoscopy center´s archives.
Concerning the procedure itself, careful instructions are provided which have medical-legal value, and are placed in the patient´s records. A copy is always kept and stored in the endoscopy center´s archives.
The hospital site where the procedure takes place, the name of the operating physician and anesthesiologist (in case general anesthesia is used), and those of the nursing staff and technicians involved are documented.
The date and time of the procedure are also carefully recorded, along with the type of anesthesia used (local or general), a description of the findings, and the therapeutic intervention undertaken.
Similarly, careful records are kept of the sterilization procedures and lot numbers of all sterilized materials (rigid bronchoscopes, forceps, etc.). Finally, special attention is devoted to tracing of the equipment which is disinfected, but not altogether sterile (such as the flexible bronchoscopes), and implantable devices (such as stents).
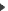 Tracing medical equipment
Tracing medical equipment
Any manipulation of medical devices adheres strictly to established protocols.
This is particularly important in regard to the disinfection and sterilization of all medical devices employed in patient care.
We divide these into two groups ; sterilizable medical devices (autoclavable), and disinfected materials (non-autoclavable).
Sterile materials are traced by lot number and date of sterilization at the hospital´s central facilities.

- Protocol (Traceability of medical devices)
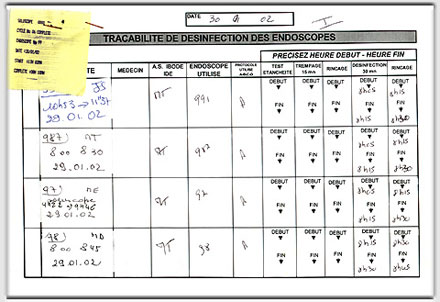
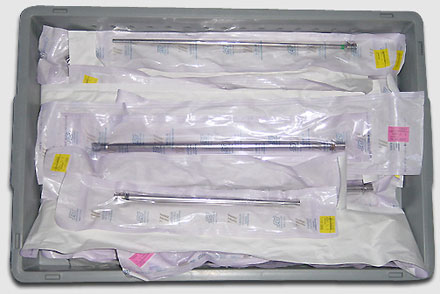
Non-sterile devices such as the fiberoptic bronchoscopes are labeled carefully following disinfection.
For each endoscope, the name of the patient, endoscopist, technician in charge of disinfection, serial number of the endoscope, and the disinfection protocol followed are documented, along with the date and time disinfection took place.
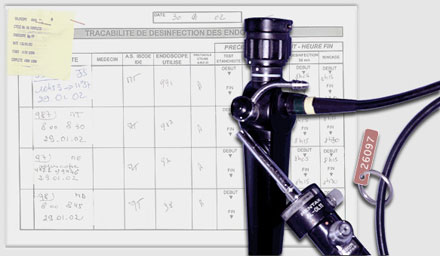
At our institution, we use metallic rings to mark clean endoscopes.
In case a washer is used, a label is provided upon which are inscribed the serial number of the endoscope, the type of procedure, and the date and time of disinfection.
All other medical devices used, such as the autoclave, the video monitors, the cameras, the light sources, and laser, are subject to close surveillance.
Careful maintenance is guaranteed by the center´s trained personnel, or by the product manufacturers´ themselves.
Either way, all maintenance protocols are carefully documented.
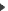 Physical plant maintenance
Physical plant maintenance
 Maintenance of the center´s installations is governed by established protocols. Routine maintenance records are updated periodically at our center.
Maintenance of the center´s installations is governed by established protocols. Routine maintenance records are updated periodically at our center.

- Protocol (Traceability of sites and procedures)
 Quality control
Quality control
 Sampling of the sterilization materials, filters, and cleaning trays, is routinely carried out by our center´s personnel, and supervised by the hospital´s quality control service.
Sampling of the sterilization materials, filters, and cleaning trays, is routinely carried out by our center´s personnel, and supervised by the hospital´s quality control service.
Several protocols are in place to assure proper maintenance of the installations and equipment.
The hospital´s central pharmacy, for example, periodically controls the expiration dates of single use perishable medical devices, such as the endoprostheses.
All quality control measures are carefully documented.




















