|
Index >
Technique > Silicone stents |
Silicone stents |
|
Silicone Stents
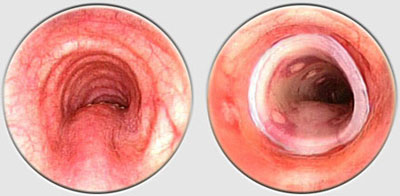
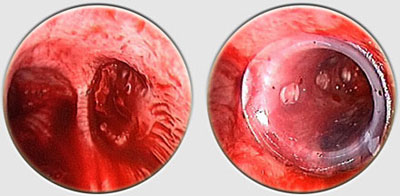
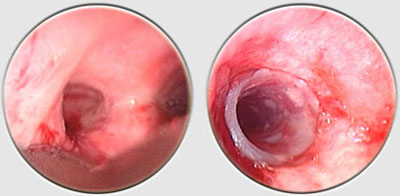
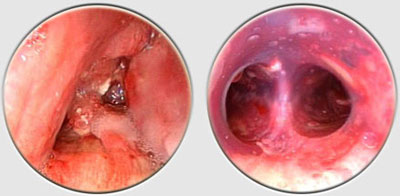
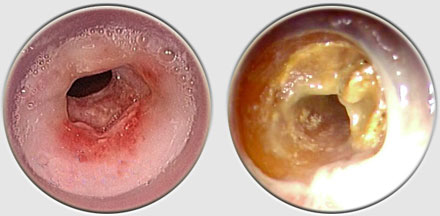
The Dumon stent® does not require tracheostomy for deployment. Its external surface is covered by silicone studs which serve a dual purpose ; avoiding migration of the stent and distributing the stent’s pressure along small sections of mucosa thereby preserving blood flow to the area. These are made from transparent silicone, which permits a view in transparency of the bronchial wall. Radio-opaque silicone leads to a loss of transparency. On a normal x-ray, a rectilinear zone in the tracheobronchial tree shows the position of the stent. On CT scan, the stent is clearly visible. The tracheal stents are generally 50 to 60 mm long (maximum 110 mm), and 14 to 16 mm in diameter (maximum 18 mm). Bronchial stents are manufactured with diameters ranging from 10 to 12 mm and lengths between 20 to 40 mm. The flexibility of the stent is also important. A stent does not need to be particularly rigid to withstand the pressure exerted by a tumor. A flexible stent is better tolerated than a rigid one. However, the stenosis absolutely must be calibrated with a rigid tube prior to placing the stent. If the stent takes on an oval shape through primary compression, it will return to a round shape within 48 hours. Additional tumor growth has never resulted in compression of a stent that is already in place. However, the tumor can continue to develop above or below the stent. This is not local but circular compression. Unlike metal stent in a bronchus, no secondary compression is possible with a silicone stent.
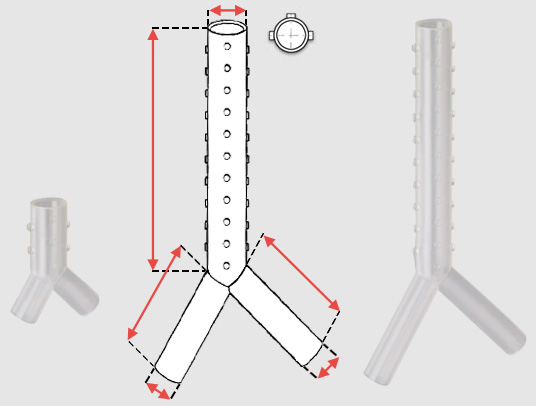
The lengths of the tracheal and bronchial limbs are variable, reaching up to 110 mm for the trachea, and 50 mm for the main stem bronchi. The right main stem limb may be fenestrated in order to facilitate right upper lobe ventilation. There are few other indications : bronchial fistula, tracheobronchomalacia…
The choice of stent depends largely upon careful measurements performed at the time of bronchoscopy. The diameter of the stent will depend on the external diameter of the bronchoscope used (yellow tube 16/15, red tube 14/13/12), while the desired stent length is measured by retracting the bronchoscope or telescope along the entire length of the stenosis. The stents must be perfectly fit to the tracheobronchial tree. To avoid complications, the shortest and widest possible stent must be used. Therefore, stenoses must always be calibrated prior to stent placement. Length : Stents should not be too long. The ideal stent completely covers the lesion and extends no more than ½ cm beyond the lesion on each side.
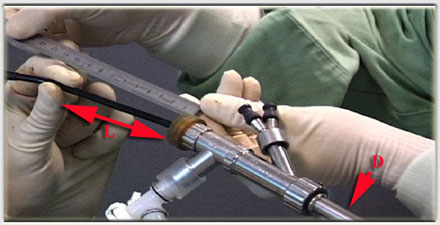
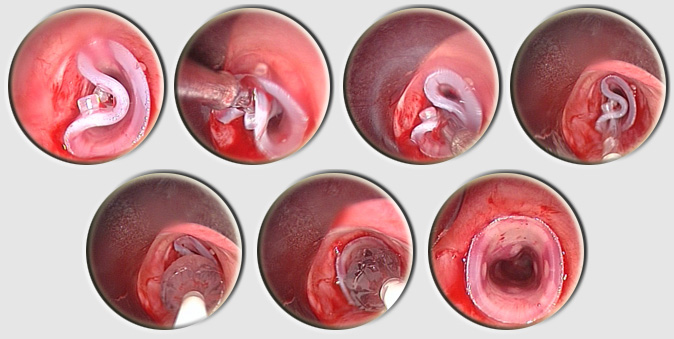
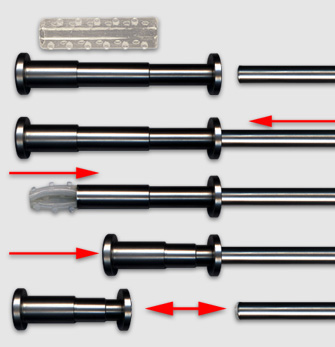
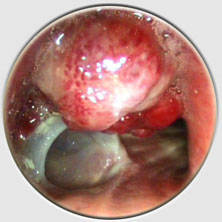
Placement is performed using a tracheal or bronchial rigid tube. The stent is lubricated with silicone and loaded into a stent applicator. The applicator is the inserted through the bronchoscope and the stent is pushed out into the trachea or the bronchus. Adjusting the stent is made by grasping the upper rim of the prosthesis ith foreign body forceps. On the left, two kind of loaders, the small for short tracheal or bronchial stent, the longer, for long Y stent or long and large straight stent. On the right two size of applicators. Applicator and loaders are color coded in function of the size of the stents. This film shows the placement of a Y stent during a live procedure (duration : 15 mn 30 s)
|
|
move
up
|
move
down
|
|
previous
|
next |